CHAPTER 20
Catastrophic Minerals
Coal is said to require 5 to 10 times its volume of trees to form, so
what process produces a coal seam more than 300 feet thick
from trees that grew on dry land?
 Coal
Coal
During an axis change, movement along the meridian of maximum change proceeds in excess of the speed of sound (in the air at sea level) and although water lags behind, the soil is removed down to bedrock. It is then replaced, or not, according to topography and the whim of the tides. Coal beds are formed from trees that may be piled more than a thousand feet high, and as the flood returns from the sea, sealed off with clay from the ocean depths. Methane (CH4) from the wood itself then converts the wood and other vegetable matter into coal. Most tree species that have been identified in coal beds grew in forests on dry land. Few grew in swamps.
Black or bituminous coal is made of trees and other plants that have been buried and sealed off from the atmosphere. In addition to methane that is generated by the wood itself, additional methane coursing through permeable sedimentary formations at times of catastrophe may be a factor in going beyond the formation of brown coal toward bituminous. Methane bubbles up from the mantle all the time, all over the world, and it floods through sedimentary formations during catastrophes.
Brown coal has a lower ‘rank’ than black coal, meaning it has less carbon content. It reflects a less complete alteration process which could be due to a less effective seal, less available methane, or a lower temperature as the chemical process progressed. Some brown coal is described as the end product of peat accumulations in muskegs or bogs, but material with this origin should be called lignite. The oldest coal deposits were formed from ferns.
Anthracite is the result of the alteration of bituminous coal by heat and pressure. It is the highest rank coal, with a carbon content as high as 98% and contains the lowest content of volatiles. This makes it favored for the production of steel. Original vegetation has a carbon content of around 50%. Bituminous (thermal) coal can be heated to produce coke. This increases the carbon content so that it can also be used for the production of steel. Volatiles driven off during the production of coke may be burned as heating gas.
 The depth of vegetation required is said to amount to 5 to 10 times the thickness of the resulting coal seam. Some coal seams exceed a hundred meters (well over 300 ft.) in thickness, and only oceanic floods are able to produce accumulations of trees and other vegetation on such a stunning scale. When water returns to the land during a flood it brings clay from the ocean depths, which can seal vegetation away from the atmosphere. It is interesting that some coal seams lie directly upon the bare rock, which tends to rule out development from a wetland.
The depth of vegetation required is said to amount to 5 to 10 times the thickness of the resulting coal seam. Some coal seams exceed a hundred meters (well over 300 ft.) in thickness, and only oceanic floods are able to produce accumulations of trees and other vegetation on such a stunning scale. When water returns to the land during a flood it brings clay from the ocean depths, which can seal vegetation away from the atmosphere. It is interesting that some coal seams lie directly upon the bare rock, which tends to rule out development from a wetland.
Sedimentary characteristics can provide additional evidence for the ways in which coal deposits are formed. In some cases, a thick seam of coal will split into thinner ones separated by sediments, and then join up again into a single seam. Vertical tree trunks (now turned to coal) sometimes extend above a horizontal coal seam. Some of these trunks are inverted, with the roots at the upper end, indicating chaotic deposition.
Coal changed our world, for it provided the energy required by the industrial revolution, transforming industry and transportation. Today we have more choices and must recognize the environmental costs of strip mining and particulate air pollution. Huge reserves of bituminous and brown coals remain untapped, but it would be desirable to replace most thermal coal with natural gas, or other types of less polluting energy.
Even though our thermal coal deposits may be sufficient to last us for more than a thousand years, there are good reasons against the ecologically destructive strip mining of bituminous and brown coal. The underground burning of thermal coal to generate coal gas offers one solution, utilizing coal seams that are too thin, or too deep, to be mined.
Crude Oil
Biogenic residues, such as fragments of kelp, are distributed widely throughout marine sediments, and physical and chemical changes by heat and pressure yield a mineraloid substance called kerogen. (Mineraloids are minerals that lack a precise chemical composition or crystal structure.) Destructive distillation of kerogen by a hypothetical thermal process that geologists call catagenesis is commonly claimed to be the process by which crude oil and natural gas are formed.
Sir Robert Robinson, a renowned British chemist, wrote in ‘Nature’ in 1963: “Actually, it cannot be too strongly emphasized that petroleum does not present the composition picture expected from modified biogenic products, and all the arguments from the constituents of ancient oils fit equally well, or better, with the conception of a primordial hydrocarbon mixture to which bio-products have been added”.
That would be Methane (CH4). I propose that all oil and deep source gas deposits are derived from methane released from the mantle. All original carbon on Earth may have come from comets, and much of it could have accompanied our primeval ocean. Carbon may persist in the mantle as the thermally stable mineral graphite, and this may be altered into methane under heat and pressure in the presence of water. Furthermore, biogenic carbon that is buried in subduction zones is known to be released into the atmosphere by volcanic activity, while large amounts of carbon remain tied up in coal deposits and limestone formations.
Methane is commonly released by earthquakes and volcanic activity. It floods through sedimentary formations where it may encounter kerogen, especially at times of catastrophism. If the encounter takes place within the oil window, a range of temperature and pressure that is well known to geologists, the methane is catalyzed and polymerized by the kerogen to produce longer hydrocarbon chains, and this is how oil is formed. The new oil tends to migrate toward the surface, with some of it being consumed by microbes along the way. Some oil will reach the surface, while some will have its journey interrupted by traps so that economic deposits of oil, often accompanied by natural gas, may be formed.
The most productive traps are associated with sub-sea ‘diapirs’ (salt domes), because there are no continental rocks to get in the way of or deflect ascending methane, and with anticlines (folds bowed upward) as in Arabia. Another common sedimentary trap is where upward trending formations ‘shale-out’ so that oil and gas are trapped by reduced porosity and permeability. Other traps commonly associated with changes to these parameters occur along former shorelines. To borrow from a first-year geology text, an incandescent light bulb has infinite porosity and zero permeability.
All crude oil tends to be chemically complex, with biomarkers retained from geological formations through which it has passed. An important consideration is the claimed ‘source rock’ where the methane was polymerized to oil. Geology books inform us that this is where oil is formed directly from kerogen, but this cannot be what occurs.
In most cases, there is not enough kerogen available, and no relationship exists in any case between the abundance of kerogen and the quantity of oil. In Arabia, large anticlines contain huge reserves of oil and are so completely full that overflow has evidently been lost to the surface. The kerogen content of the sediments, however, is less than that of many fields elsewhere.
Oil Sands and Oil Shales are made up of oil that has been degraded by contact with water, together with sand or mud. Tar pits at La Brea in California have been dug on a sub-cropping formation of salt water-degraded oil from a major oil spill along the coast. A large number of animal bones are included at La Brea because oceanic Flood waters swept away so many animals from the continent, 10,500 years ago.
Natural Gas
Methane from the mantle continually percolates upward along continental shelves to form methane hydrate, consisting of an icy slush. It is technically a clathrate, which is a lattice structure of one molecule containing molecules of another molecule. In this case, the lattice structure is formed of water, containing just over 4% methane. The sea-bed temperature tends to maintain itself within a narrow range which is below the freezing temperature of fresh water and above that of salt water. Methane hydrate is kept solid by temperature and by hydrostatic pressure. The hydrate, which acts like ice, forms an impermeable seal close below the seabed, trapping rising methane gas beneath it. This can be hazardous to drilling operations being conducted before blowout protectors have been installed. Any sudden release of methane gas could result in a fire, or destroy the buoyancy of a drillship, causing it to list or even sink. Ships have sunk from loss of buoyancy due to gasses released from the sea-bed. Blowout protectors enable a drill crew to ‘shut-in’ a well and control any gasses encountered.
Some stories from the Bermuda Triangle, and elsewhere, involving ships sunk at sea and pilots disorientated from faulty magnetic compass readings, are attributable to releases of methane. A landslide on the continental shelf, which is a common occurrence, may destroy a methane-hydrate cap so that a cloud of methane gas is suddenly released. As the gas escapes it reduces buoyancy in the water and creates random local magnetic fields due to static electricity.
Methane deposits occur in combination with salt water, and with gasses including carbon dioxide, nitrogen, hydrogen sulfide (which is a deadly poison) and sometimes helium. Helium results from the decay of radioactive minerals such as uranium and potassium in granitic basement rocks, below the sediments. Some wells, such as in Saskatchewan, contain enough helium to be drilled for its production. The most common constituent of natural gas is usually methane, but processing of natural gas by fractional distillation may yield ethane, propane and many heavier components. These products are used for heating, transportation, electricity generation and manufacturing feedstocks, especially for plastics.
A deep source of methane is indicated by peaks of short-lived, radioactive radon gas emanating from the ground during earthquakes. Radon arrives just ahead of methane peaks, and it is concluded that the deep-sourced radon, some of which has an extremely short half-life, has been swept ahead by methane. This could provide warning of an impending earthquake. Fires can erupt at the surface along faults during earthquakes, as in California, and the sea has been observed on fire off the coast of Chile after an earthquake. This would be the result of methane mixed with air, set on fire by static electrical discharges.
Since some lubricating fluid must be present to enable earthquakes to occur at depths where rocks have a plastic quality and would otherwise slowly deform, liquid methane is a prime suspect. Compressed gas under intense pressure can escape along a fault. Rapidly expanding gas from beneath the sea can displace a great volume of seawater, which can result in a tsunami. This may be the way in which most tsunamis form, as some faults concluded to have been the cause of earthquakes have been found on examination to have undergone little or no vertical displacement.
I am proposing that economic gas deposits form as a result of methane percolating up from the mantle, without any relationship to kerogen. This is despite the release of biogenic methane often observed in wetlands, where rising streams of methane bubbles burn readily. In winter, biogenic methane may accumulate beneath ice formed on ponds.
Most carbon on Earth may reside in the mantle, from where it is released directly, while biogenic carbon that has been subducted also recycles back to the atmosphere. In either case, carbon can become trapped on its way to the surface. Thomas Gold promoted the investigation of deep gas deposits which promises a practically inexhaustible source of energy. Natural gas and its derivatives have been generally transported in (relatively) low-pressure pipelines, but are increasingly shipped overseas as liquefied natural gas (LNG) in pressurized containers on dedicated tanker ships.
Fossil Fuel
Fossil Fuels are a favorite topic for climate-change alarmists these days, especially regarding how quickly we are using them up and what a threat they present to our environment. Their origin is taken for granted to be decayed plant and animal matter. Since ‘Wikipedia’ can generally be taken as an example of accepted orthodoxy, let’s see what it has to say.
Wikipedia informs that “A fossil fuel is a fuel formed by natural processes, such as anaerobic (out of contact with free oxygen) decomposition of buried land organisms containing energy originating in ancient photosynthesis.” Photosynthesis is the process employed by plants to use sunlight to convert carbon dioxide and water into sugars essential for their growth.
I think there would be full agreement that “… originating in ancient photosynthesis” applies to coal, but if this is the definition of fossil fuels that we are required to accept, then coal is the only fossil fuel. Granted, we would have to include some natural gas from plant material that has been buried. Otherwise, coal is it!
I accept the belief of the late Thomas Gold that Earth’s mantle contains an immeasurable quantity of dissolved methane. This is the source of methane that constantly bubbles up to the surface all over the world. It is the source of methane that becomes trapped in rock strata and salt domes all over the world, and it is the source of methane that is catalyzed and polymerized by the mineraloid kerogen into crude oil all over the world.
Since the only part played by photosynthesis in this process is in the formation of the kerogen, which is simply a catalyst (a substance that induces a chemical change without itself undergoing a permanent change), to call either natural gas or crude oil a “fossil fuel” is inappropriate. Furthermore, to base a carbon-tax scheme on such a flimsy political premise as this is an example of how our society frequently functions out of contact with reality. I think I have made it abundantly clear that we have to become better stewards of the Earth, but an important part of this is going to require getting the science right.
Older Australians will recall an award-winning program aired on ABC-TV in the early 1970’s called ‘The Aunty Jack Show’, which attained instant cult status. One episode included a group of teenage girls whose repeated mantra was “Thirteen high-school girls can’t be wrong!” This sometimes comes back to me as I ponder the scientific wisdom of some of our political leaders.
Base Metals
The richest massive-sulfide deposits containing copper and zinc, with gold and other useful metals, began at thermal vents along a mid-ocean ridge. There, bacteria are the first step in concentrating trace amounts of metal that are leached out of volcanic rocks by circulating hot acidic water. After successive episodes of geological activity, which include burial, concentration, and uplift, accessible deposits occurring on land may form and be mined. Some of the world’s richest ore deposits were formed in this manner, as a distant result of the energy imparted by the impact of asteroids.
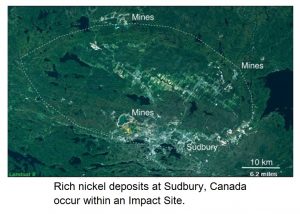 One of the world’s richest nickel deposits, occurring within an impact site at Sudbury in Canada, may have been the result of a nickel-rich asteroid, with metal sulfides now intruded into the rocks as veins by post-impact mobilization.
One of the world’s richest nickel deposits, occurring within an impact site at Sudbury in Canada, may have been the result of a nickel-rich asteroid, with metal sulfides now intruded into the rocks as veins by post-impact mobilization.
Placer Gold
Even alluvial (placer) deposits can be enriched during a catastrophe, as in the Klondike, where a gentle jigging action, as water sloshed back and forth in the Arctic Basin following an axis change, may have been responsible for a fabled paystreak of gold along Eldorado Creek. In fact, there is no type of mineral deposit on Earth that has not resulted, either directly or indirectly, without catastrophic involvement. This means either asteroid impact or from the close proximity of another world.
 The Matson Creek gold deposits in the Yukon are of particular interest to me. From my own work there, I know that visible gold in quartz veins does not occur. Most gold in the bedrock occurs as a few parts per million in small crystals of pyrite (FeS2), disseminated through a common type of metamorphic rock called schist. This rock is made up entirely of mica flakes and quartz grains, and can often be scuffed away by a boot, indicating how susceptible it is to erosion.
The Matson Creek gold deposits in the Yukon are of particular interest to me. From my own work there, I know that visible gold in quartz veins does not occur. Most gold in the bedrock occurs as a few parts per million in small crystals of pyrite (FeS2), disseminated through a common type of metamorphic rock called schist. This rock is made up entirely of mica flakes and quartz grains, and can often be scuffed away by a boot, indicating how susceptible it is to erosion.
The schist may have been altered from shale that was formerly mud, with the gold being disseminated throughout the mud. In turn, the mud may have resulted from weathering of igneous or volcanic rock, as one geological cycle followed another. Under a fairly arid climate, pyrite breaks down and the contained gold is taken up by acidophilic (acid-loving) bacteria and deposited deeper, at the limit of oxidation, as native (visible) gold for the first time. Nuggets of placer gold typically contain roughly 90% gold and 10% silver, with up to 1% copper.
The gold is washed into streams, and in the famous case of Eldorado Creek, appears to have been concentrated into the fabulous paystreak by a jigging action of the Arctic Ocean as it sloshed back and forth within its basin. That was before permafrost conditions descended on the region. At Matson Creek, which is underlain by permafrost, an east-west trending stream in a broad valley has migrated northward from differential melting along the south-facing (sunny) bank. Gold once widely dispersed across the north-facing slope had been concentrated into economic deposits by normal stream action.
 Diamonds in Kimberlite
Diamonds in Kimberlite
Diamonds occur in vertical pipes, from deep within the crust. This raises the question of how the diamonds formed, and what force drove them up to the surface through several miles of solid rock. The richest diamond pipes consist of kimberlite, which is an uncommon rock type rich in carbonates and ultrabasic minerals, especially olivine. The green gemstone, peridot, is the gem version of olivine.
When Earth comes into close contact with another world, its continents are attracted upward and weigh less than usual. This provides opportunity should the right materials accumulate beneath the continent. When the other world has passed by, the weight of an entire continent is brought down upon this material which is driven vertically through miles of solid rock to the surface.
The pipe structures tend to be carrot-shaped because they encounter water close to the surface which produces steam, and may be accompanied by significant amounts of other gasses. They burst with great force as they reach the surface, considerably enlarging their diameter. Once again, catastrophism is the reason.
If the material beneath the continent is rich in carbon, diamonds may form, to be carried up rapidly with the kimberlite to reach a cooler environment before they can revert to graphite. In one instance, two broken diamonds were found on widely separated levels of a South African mine and were found to fit perfectly together, having been broken during their passage. Most known kimberlite pipes are very old and date back to Precambrian times.
One aid in prospecting for diamonds is a small number of ‘indicator minerals’ which always accompany the diamonds. The most important of these is a red garnet containing chromium, called pyrope. Since diamonds have been found containing inclusions of pyrope, their conditions of formation must be very similar, with the pyrope forming first. In certain environments, ants have been known to carry small garnets up from underground, thereby assisting in the prospector’s search.
Kimberlite pipes would normally provide an intense magnetic expression, but this can be reduced by deep weathering into clay called blue ground. Above the blue ground, a water degraded clay zone called yellow ground may be present, frequently mixed with alluvium.
Diamonds in Lamproite
Other pipes, some of which also contain diamonds, are made of a rock called lamproite, which is typified by a characteristic suite of minerals. In this case, the minerals are rich in potassium and magnesium and again include olivine. Like kimberlites, lamproites weather into soft secondary minerals, including talc, chlorite and serpentine.
Lamproite is notable for containing a wide range of metals, together with some quite unusual minerals. These pipes tend to be younger, from shallower depths, and have attracted considerable interest by geologists in recent years. While the emplacement of lamproite formations appears to lack the extreme violence of kimberlites, they have many attributes in common.
Sapphires
Gemstones are naturally attractive to most of us, and just as diamonds form under catastrophic circumstances, so do sapphires. One reason for including sapphires here is because I was involved with mining sapphires in the state of New South Wales in Australia. The deposits we mined were all alluvial and typically consisted of cobble-sized (fist-sized) granitic rocks, termed “wash” by the miners, with thick sandy clay containing loose sapphires. The most productive areas are close to contacts between the granite bedrock and overlying volcanic basalt.
The reason for this is that the sapphires were introduced in explosive volcanic activity (which geologists call “phreatic events”) and covered by thin flows of basalt lava which are now weathering away. The depth of origin was much less than that of kimberlites, but similarly, groundwater turned to steam contributed to the violence of the event. Unusual occurrences like this don’t just happen. Volcanic activity increases greatly at times of catastrophe and produces results that cannot be explained within uniformism.
The wash was mined along present, and former, stream courses using backhoes and passed through trommels (rotating screens with water spray bars), and jigs, which use water in a pulsing action to concentrate minerals by their higher specific gravity. In northern New South Wales, almost all sapphires are blue, being darker than those from Sri Lanka due to higher trace iron content. In central Queensland, to the north, many sapphires are particolored, meaning blue, yellow and green.
Sapphires are dichroic which means the color varies depending on whether light is traveling parallel to, or across, the long axes of the hexagonal crystals. Whenever significant quantities of a gemstone are discovered that fail to meet the norm for color or other properties, marketing initiatives generally follow to convince buyers that the new type is just as desirable, or even more so than the accustomed ones. Although I have sold only blue sapphires, I have to agree that particolored sapphires can be beautiful.
Red sapphires are rare, and any with the appropriate hue would be classified as rubies, while all other colors are called “sapphire”. Sapphires and rubies are the gem version of the mineral corundum (Al2O3), which is second in hardness to the diamond on Mohs’ scale, which is an empirical scale of hardness, from 1 to 10, known to every geologist and prospector. The specific gravity of sapphires is about 4, which is 1 ½ times that of quartz sand, explaining why jigs are an effective means of recovery. Sluice boxes require a greater difference in specific gravity except for feed with a narrow size range and closely controlled water flow.
 Evaporites
Evaporites
Salt (i)
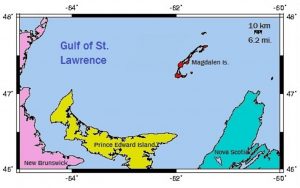
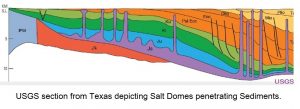
Salt in the form of common table salt (NaCl) is the most common evaporite. It settles out of boiling brine over the lava pool of a major impact site when there is limited access by the sea. A huge volume of seawater can be evaporated over a lava pool, resulting in a thick deposit of pure salt. This salt is coarse-grained, clear and crystalline. At Prince Edward Island, where Shell Oil Company drilled vertically through 3 kilometers of salt, the question arises whether this represents the thickness of the original bed.
Allan O. Kelly, writing in ‘Impact Geology’, explained how Prince Edward Island has been formed out of four major pieces that drifted onto the lava pool from the south rim of the impact site. A salt layer could have been disturbed by the movement of overlying formations by this action, and a diapir (salt dome) may have resulted. This could explain the great thickness of salt encountered by Shell. Perhaps P.E.I. will again present an inviting target for oil exploration.
 The Magdalen Islands, not far north of P.E.I. but part of the province of Quebec provides another example of a small archipelago that floated upon the same lava pool. That lava pool resulted in the Gulf of St. Lawrence, and the Magdalen Islands are also underlain by massive deposits of salt. This archipelago displays similarities to the Belcher Islands in Hudson Bay, and may similarly represent a portion of the crater rim that floated upon the lava pool.
The Magdalen Islands, not far north of P.E.I. but part of the province of Quebec provides another example of a small archipelago that floated upon the same lava pool. That lava pool resulted in the Gulf of St. Lawrence, and the Magdalen Islands are also underlain by massive deposits of salt. This archipelago displays similarities to the Belcher Islands in Hudson Bay, and may similarly represent a portion of the crater rim that floated upon the lava pool.
Salt (ii)
Other salt deposits form on the surface under much cooler conditions, from evaporation by the sun. These deposits display horizontal bedding, and in a climate that is sufficiently warm and arid, and a coastal lagoon that has just the right geometry (requiring a shallow sill at the entrance to restrict the entrance of sea water) the salt beds may alternate with thinner beds of potash. Potassium is the sixth most abundant element in seawater, and most potash evaporites form simple compounds as chlorides and sulfates. These commodities are valuable to agriculture as well as to industry.
As studies by the U.S. Department of Mines have shown, basin shape and size can be critical. Under certain circumstances, a range of evaporites will form within the same long and narrow basin, from limestone, then gypsum, and then salt. When the brine has been sufficiently concentrated by evaporation, potash will form. The very last fluid concentrate from the sea water consists of bitter salts, which include elements such as iodine and bromine, which are so resistant to evaporation that they are rarely incorporated into deposits.
Catastrophism might appear at first to have played no role here. In the formation of basins and variations in sea level, however, and in the determination of latitude by axis change, so that deposits may persist at latitudes where they could not have formed, catastrophism is always there in the background, making a difference.
Minerals and Evolution
Every point on Earth’s surface must have existed in the Tropics and in the Polar Regions repeatedly, and after every axis change our planet sought to regain its preferred form. As it did so rocks were squeezed and stretched and folded and fractured, and mobile rock intruded into less mobile rock. Hot, aqueous solutions dissolved minerals from the rock and concentrated them into mineral deposits with the active assistance of bacteria. The means by which the intrinsic mineral wealth of our planet has become available to us is through asteroid impact and bioactivity.
The influence of impacts exceed even that, for every major impact has turned over another leaf in the book of evolution on Earth. Biologists consider that 99% of all the species that have ever lived on Earth no longer exist. Many became extinct as a result of an oceanic flood from an axis change, brought about by the impact of a large asteroid. Extinction provided the opportunity for new species to evolve, including us.
Every continent developed from the accumulation of smaller parts: volcanos generated along subduction zones, volcanic flows and granite batholiths, sediments deposited by oceanic floods, and terranes from afar. Asteroid impacts have formed almost entire continents and made their minerals available to us. Oceanic floods produced our topsoil, but elsewhere removed it so that minerals in the rocks would be more readily discovered. As the major driving force of evolution, asteroid impacts have made Earth unique within our Solar System, and perhaps anywhere.
Gift of Catastrophism
Our Earth has an oblate form, which means that it is flattened at its geographic poles. The amount of flattening is considerable, amounting to 21 km (13 mi.) at each pole. With every axis change, Earth seeks to return to an ideal form for its speed of rotation, and in doing so rocks are fractured and melded so that most outcrops anywhere in the world have been extensively fractured. Volcanos become active at such times, while deep beneath the surface hot liquids and gasses surge through permeable formations.
When continents plunge beneath the oceans, water scours their surface down to bedrock, river valleys are deepened, and ice caps that terminate within a continent are destroyed. Methane from the mantle flows through sedimentary rocks, and deposits of coal and crude oil and natural gas are formed. Hot liquids, often acidic, are on the move, dissolving metals at one place and depositing them elsewhere when a change in the chemical environment is encountered.
Metals disseminated in the rocks are influenced by electrical gradients as well, causing rich concentrations of metallic minerals, such as gold, to be deposited in quartz veins. It is during catastrophes that most geological activity takes place. Then, things quieten down until the next time. Between catastrophes, the most intense geological activity is volcanic.
One cannot bring to mind a useful mineral or rock that has not been made available to us through catastrophism. Deposits of petroleum, base metals, industrial minerals, gemstones and placer gold, and rock mined in quarries, and sand, are either formed directly or influenced in some manner by the processes of catastrophism.